Ovarian tissue from Chinese hamsters is used to create human ova. They find widespread use in the biotechnology industry, particularly in the manufacturing of biopharmaceuticals. To produce therapeutic compounds and complicated proteins, CHO cells, which are mammalian cells, have various benefits. Due to their ability to express recombinant proteins at high levels and undergo post-translational modifications such glycosylation, CHO cells are finding increasing use. Biologics such as monoclonal antibodies, hormones, enzymes, and cytokines are often produced using CHO cells due to their favourable characte ristics. The economics of CHO cell production and its ultimate application are the focus of the Chinese hamster ovary cells industry.
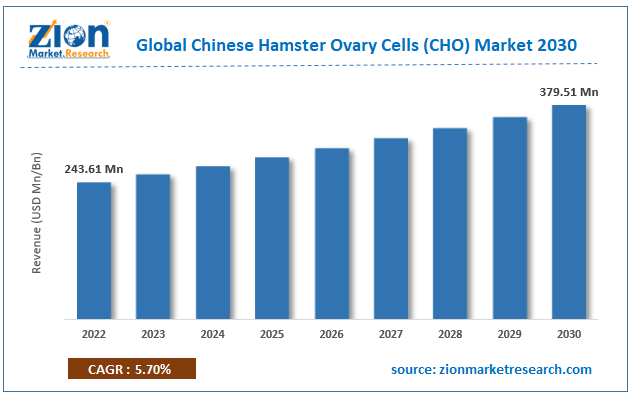
With the rising number of patients and the lack of sufficient effective medications or therapies for treating a wide range of critical conditions like autoimmune diseases, cancer, and others, the global Chinese hamster ovary cells (CHO) market is expected to rise to meet the rising demand for quality patient care. CHO cells are favoured because they have the machinery for proper protein folding, post-translational modifications, and assembly, making them ideal for expressing and generating recombinant proteins. These characteristics make them ideal for mass synthesis of complicated therapeutic proteins.
Extensive research has been conducted on CHO cells over the years, and after decades of trial and error, researchers have found optimal conditions for these cells. Due to the extensive R&D that has been done over a longer period of time, scientists now have a comprehensive grasp of CHO cell physiology, genetics, and culture conditions. Having access to a healthy CHO cell line further helps the Chinese hamster ovary cells market. The sector’s revenue is boosted even further by the rising demand for biosimilars, innovative new technologies, and expanding regulatory approval.
As the use of CHO cells in biopharmaceutical production is often subject to intellectual property constraints and licencing agreements, this could pose a challenge to the expansion of the worldwide Chinese hamster ovary cells market. Due to patents, some cell lines and the technology that go along with them may be inaccessible to all, driving up the price for businesses that want to employ them. In addition, dealing with the complexities of CHO cell use regulation can be challenging, particularly in multinational contexts. Another factor contributing to sluggish CHO cell industry expansion is the widespread use of alternative expression systems such as yeast, bacterium, and plant-based systems.
Chinese Hamster Ovary Cells (CHO) Market: Competitive Analysis
The global Chinese hamster ovary cells (CHO) market is led by players like:
- Lonza Group Ltd.
- Thermo Fisher Scientific Inc.
- Merck KGaA
- Sartorius AG
- Catalent Inc.
- WuXi Biologics
- GE Healthcare Life Sciences
- Samsung Biologics
- Boehringer Ingelheim BioXcellenc
- MilliporeSigma
- Fujifilm Diosynth Biotechnologies
- GenScript Biotech Corporation
- AGC Biologics
- Selexis SA
- Rentschler Biopharma SE
- Celonic AG
- Abzena Ltd.
- ProBioGen AG
- BioVectra Inc.
- Eurofins BioPharma Product Testing
- Glycotope GmbH
- Horizon Discovery Group plc
- Invetech
- Lonza Biologics
- Fujifilm Irvine Scientific.
The global Chinese hamster ovary cells (CHO) market is segmented as follows:
By Application
- Clotting Factors
- Monoclonal Antibodies
- Hormones
- Cytokines
- Fc-Fusion Protein
- Enzymes
- Others
By Product
- CHO-DG44
- CHO-K1
- CHO-S
- CHO-DXB1
- Others
By End-User
- Contract Development & Manufacturing Organization
- Biopharmaceutical Companies
- Academic Institutes & Research
- Biotech Companies
- Clinical Research Organizations
- Others
By System
- Metabolic Selection System
- Antibiotic Selection System
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa


